 The ongoing COVID-19 pandemic poses increasing challenges for the healthcare industry. COVID-19 diseases must be detected immediately in order to stop their spread by the virus. It is therefore important that blood tests are diagnosed as soon as a blood sample is taken. The use of mobile and stationary diagnostic systems at the point of care represents an innovative solution for the immediate diagnosis of illnesses. Microfluidic test strips, a standardised workflow and connectivity to the cloud in the hospital IT infrastructure provide real-time results. Certain parameters allow the delivery of precise results equivalent to stationary laboratory tests.
The ongoing COVID-19 pandemic poses increasing challenges for the healthcare industry. COVID-19 diseases must be detected immediately in order to stop their spread by the virus. It is therefore important that blood tests are diagnosed as soon as a blood sample is taken. The use of mobile and stationary diagnostic systems at the point of care represents an innovative solution for the immediate diagnosis of illnesses. Microfluidic test strips, a standardised workflow and connectivity to the cloud in the hospital IT infrastructure provide real-time results. Certain parameters allow the delivery of precise results equivalent to stationary laboratory tests.
► Equipment of Point-of-Care Diagnostic Systems with RFID Technology
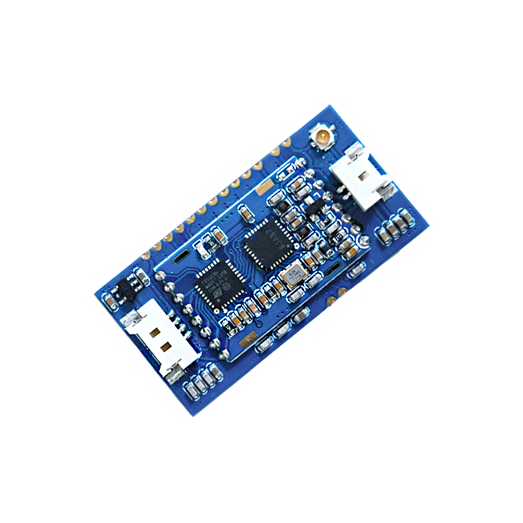
The Integration of an RFID OEM Module in diagnostic systems validly identifies the batch packaging of the respective test. RFID OEM Module M890 operates in the high frequency range of 13.56 MHz and validly reads out all data in the close range of up to 8 cm. After successful tag authentication, all test parameters are stored in the system. The pre-defined test parameters and all patient data can be written to the RFID OEM module. This enables the transfer to the patient file immediately.
More Information: https://en.idtronic-rfid.com/embedded-rfid-modules/hf/module-m890
► Attaching RFID Tags to Batch Packs

In order for the data on the microfluidic test strips to be read, corresponding RFID tags are required on the batch packaging. The calibration of the correct test strip is important for the exact definition of the test parameters. Special RFID tags with a permanently usable adhesive strip are suitable for this application. Adhesive Labels offer a matt white surface that protects the chip and the antenna underneath from liquids.
Weitere Informationen: https://www.idtronic-rfid.com/f/idtronic_adhesive-labels.pdf
► Case Study: Rapid Laboratory Diagnostic Test of Blood Samples

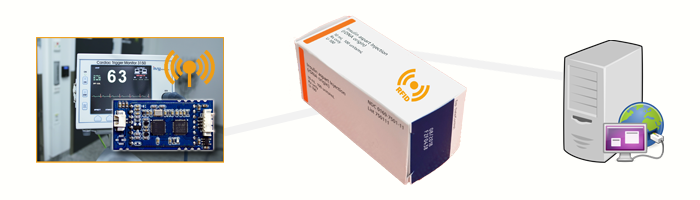
In laboratory environments or hospitals, rapid tests are an important solution for acute cases of illness. Embedding an RFID OEM Module into an existing device is very easy due to the single-sided components. The corresponding soldering pads on the underside of the RFID module can be firmly attached to all surfaces. The batch packages with the adhesive labels can be written with a unique UID and stored data. The unique UID allows the batch packages to be assigned to the microfluidic test strip. As soon as a blood test is performed, the desired test strip must be selected. The batch package is identified by placing it on the RFID OEM module. The connection of an RFID Antenna to the RFID module enables the specific identification of the RFID Tag. The device lists all possible test parameters for the test. The medical staff inserts the test strip with the blood sample into the device. The blood is analysed directly in the device. Through communication with external data management systems, a transfer directly to the patient file is possible. The assignment of the patient to the test can be done by means of a barcode reader. The barcode reader scans the data on a barcode beforehand and transmits it to the system. This enables the automatic assignment to the patient – without any further administrative activities.
► Contact our sales managers and let them advise you:

Contact Person for Product Requests
Patrick Kochendörfer
– Senior Product Manager –
Phone: +49 621 66900 94-21
E-mail: patrick.kochendoerfer@idtronic.de
 Contact Person for Media Requests
Contact Person for Media Requests
Maria Mahler
– Marketing Manager –
Phone: +49 621 66900 94-11
E-mail: maria.mahler@idtronic.de
